Xiaoheng Liu's group recently published research papers in the journals Genes & Diseases and Theranostics. By exploring the mechanobiological mechanism of atherosclerosis, they proposed targeted and responsive nanoparticles based on the mechanosensitive protein CTSK.
Atherosclerosis (AS) is a chronic inflammatory disease that occurs at bifurcations and bends of blood vessels and manifests as abnormal hemodynamic changes, such as disturbed flow and flow separation. Previous studies have confirmed that disturbed flow at atherosclerotic sites could up-regulate the expression of various proteases, which in turn degrade extracellular matrix components such as elastic fibers and collagen, leading to endothelial dysfunction and vascular remodeling. The research group demonstrated whether cathepsin K (CTSK), an important mediator of degrading extracellular matrix proteins, responds to the regulation of disturbed flow and promotes the occurrence of atherosclerosis at first, and then elucidated the mechanobiological mechanism of disturbed flow regulates CTSK expression and mediates atherosclerosis. On this basis, a CTSK enzyme-responsive targeted nano-delivery system was designed to treat atherosclerosis.
It has been found that disturbed flow could mediate vascular endothelial inflammation through the Integrin-cytoskeleton-NF-ĸB signaling axis, thereby affecting the development of atherosclerosis. The related results were published in the journal Genes & Diseases (Cathepsin K contributed to disturbed flow-induced atherosclerosis is dependent on Integrin-actin cytoskeleton-NF-κB pathway, April 25, 2022).
The research group constructed anin vivodisturbed flow model by local ligating mice's left common carotid artery. The results confirmed that disturbed flowin vivoaccelerated the formation of atherosclerotic plaques in ApoE -/-mice, with the increased diameter of the left common carotid vessels, increased intimal thickness, and rupture of the elastic layer (Figure 1).
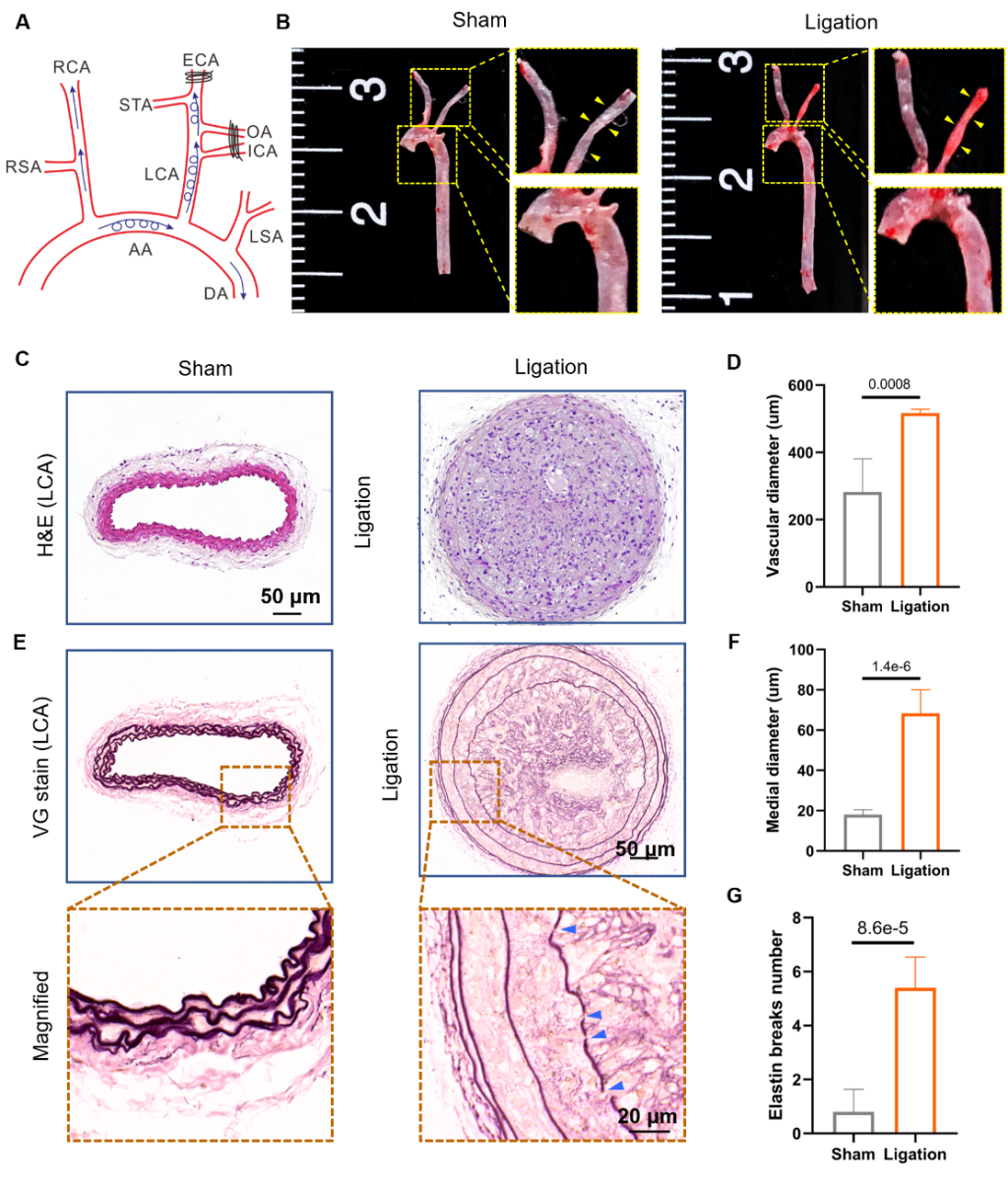
Figure 1. Atherosclerosis and vascular remodeling induced by disturbed flowin vivo
The results ofen faceimmunofluorescence staining showed that the expression of CTSK in vascular endothelial cells of the disturbed flow area (LCA, AA) was significantly higher than that in the laminar flow area (RCA, DA) (Figure 2A). Furthermore, CTSK protein and mRNA levels were significantly up-regulated in disturbed flow regions (LCA, AA)in vivocompared with laminar shear stress regions (RCA, DA) (Figure. 2B&C).
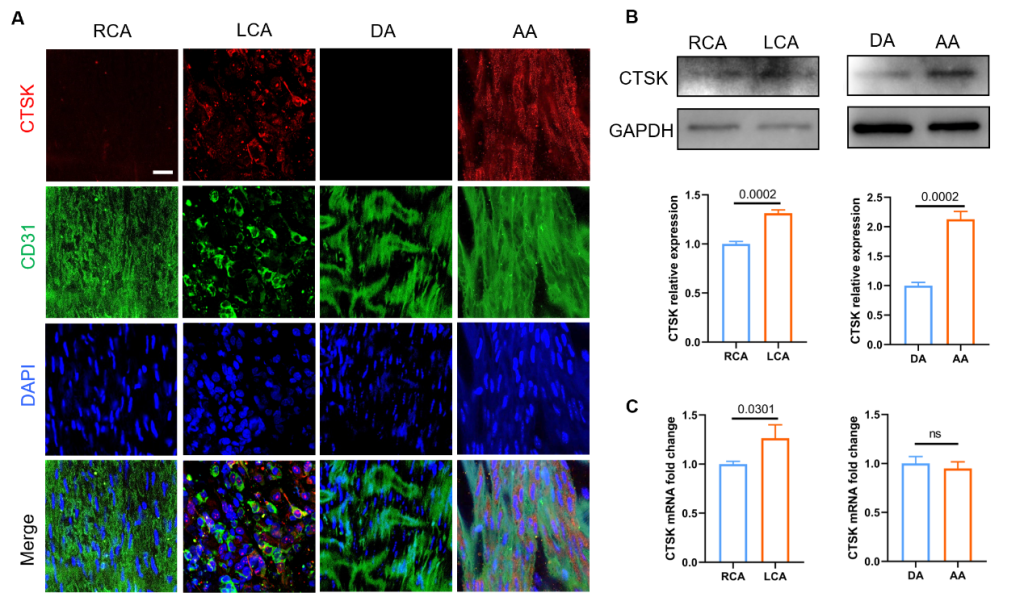
Figure 2. The expression of CTSK was up-regulated by disturbed flowin vivo
By blocking the mechanoreceptor Integrinin vivo,it was found that atherosclerosis caused by disturbed flow was alleviated. In addition, the expression of CTSK and vascular remodeling induced by disturbed flow was significantly decreased after Integrin blocking (Figure 3).
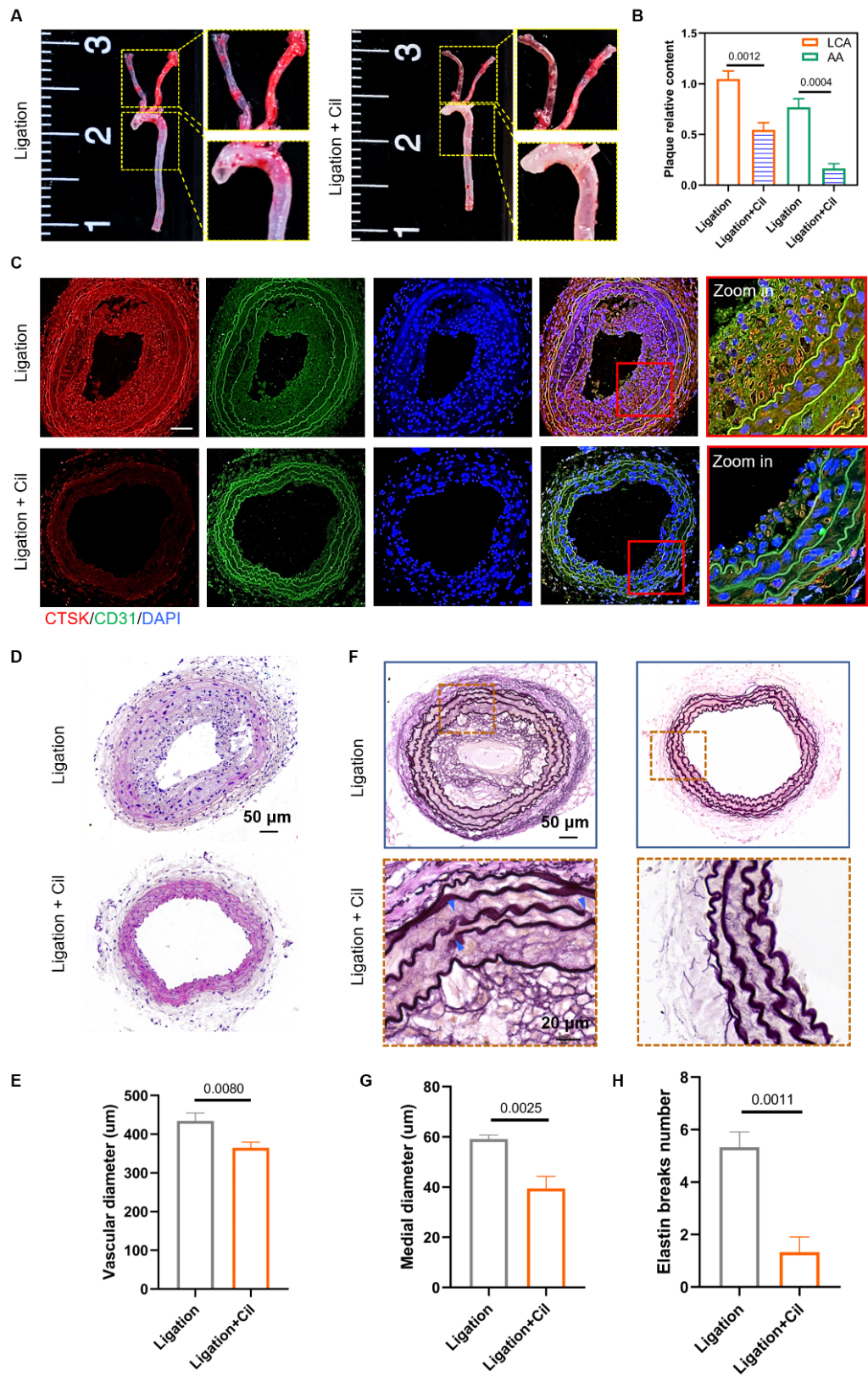
Figure 3. Integrin blocking alleviates atherosclerosis and vascular remodeling induced by disturbed flow
On this basis, the research group constructed a target-responsive nanoparticle based on the mechanosensitive protein CTSK to treat atherosclerosis. The results were published in the journalTheranostics (Inflammatory endothelium-targeted and cathepsin responsive nanoparticles effectively against atherosclerosis, May 16, 2022).

The research group designed and synthesized atherosclerosis-targeting and CTSK enzyme-sensitive block polymers PLGA-PEG-c(RGDfC) and PLGA-Pep-PEG, and combined the two polymers with the immunosuppressant rapamycin ( RAP) to prepare drug-loaded nanoparticles RAP@T/R NPs. The characterization results (Figure 4) showed that the T/R NPs were uniform spherical, with a particle size of about 274 nm, and had goodin vitrostability. RAP@T/R NPs could rapidly release drugs in the presence of the CTSK enzyme.
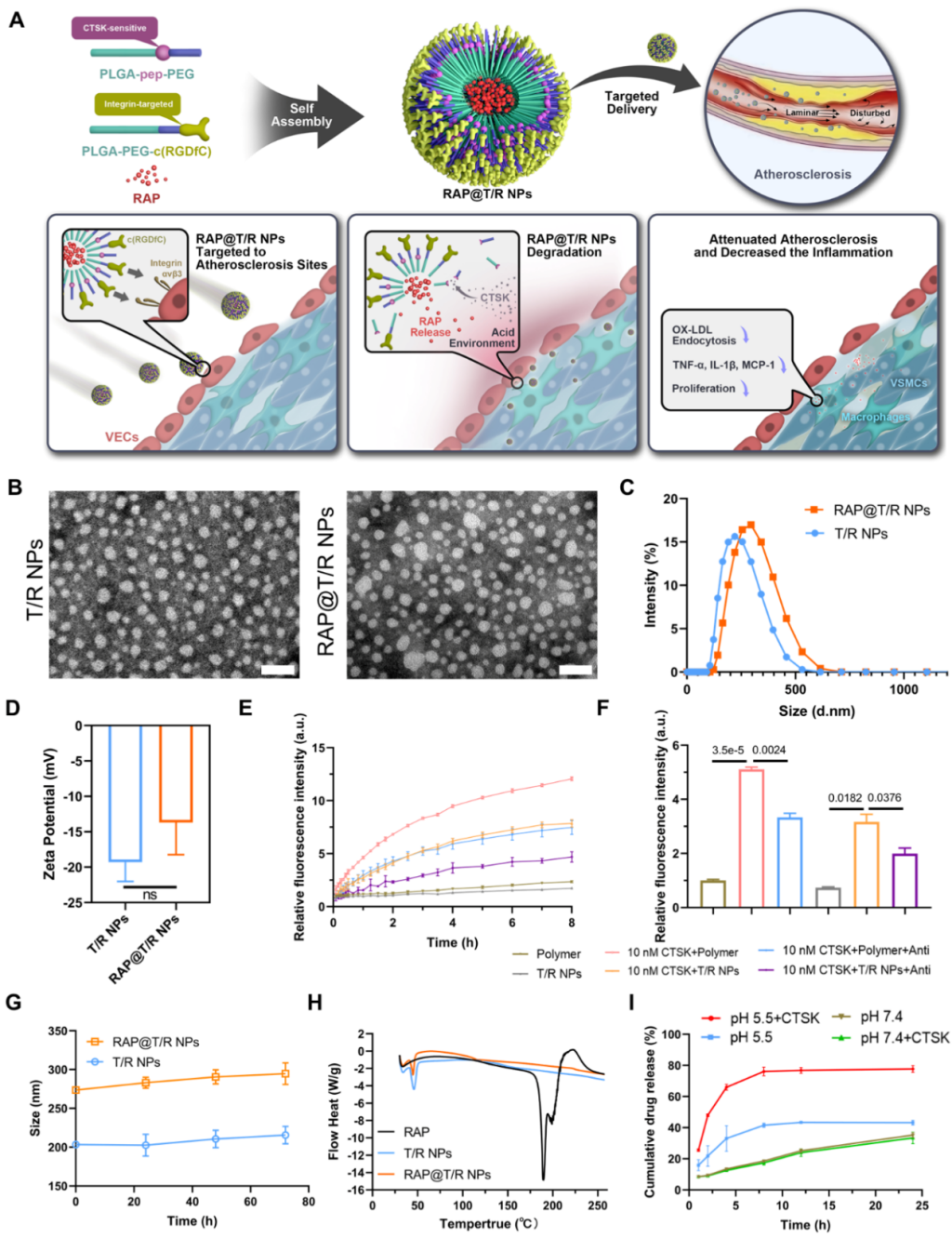
Figure. 4 Schematic diagram andin vitrocharacterization of target-responsive nanoparticles for the treatment of atherosclerosis
Thein vitrotreatment effect showed that RAP@T/R NPs could inhibit the phagocytosis of oxidized low-density lipoprotein by inflammatory macrophages, inhibit the release of inflammatory factors TNF-α, IL-1β and MCP-1, and inhibit the proliferation of macrophages ( Figure 5).
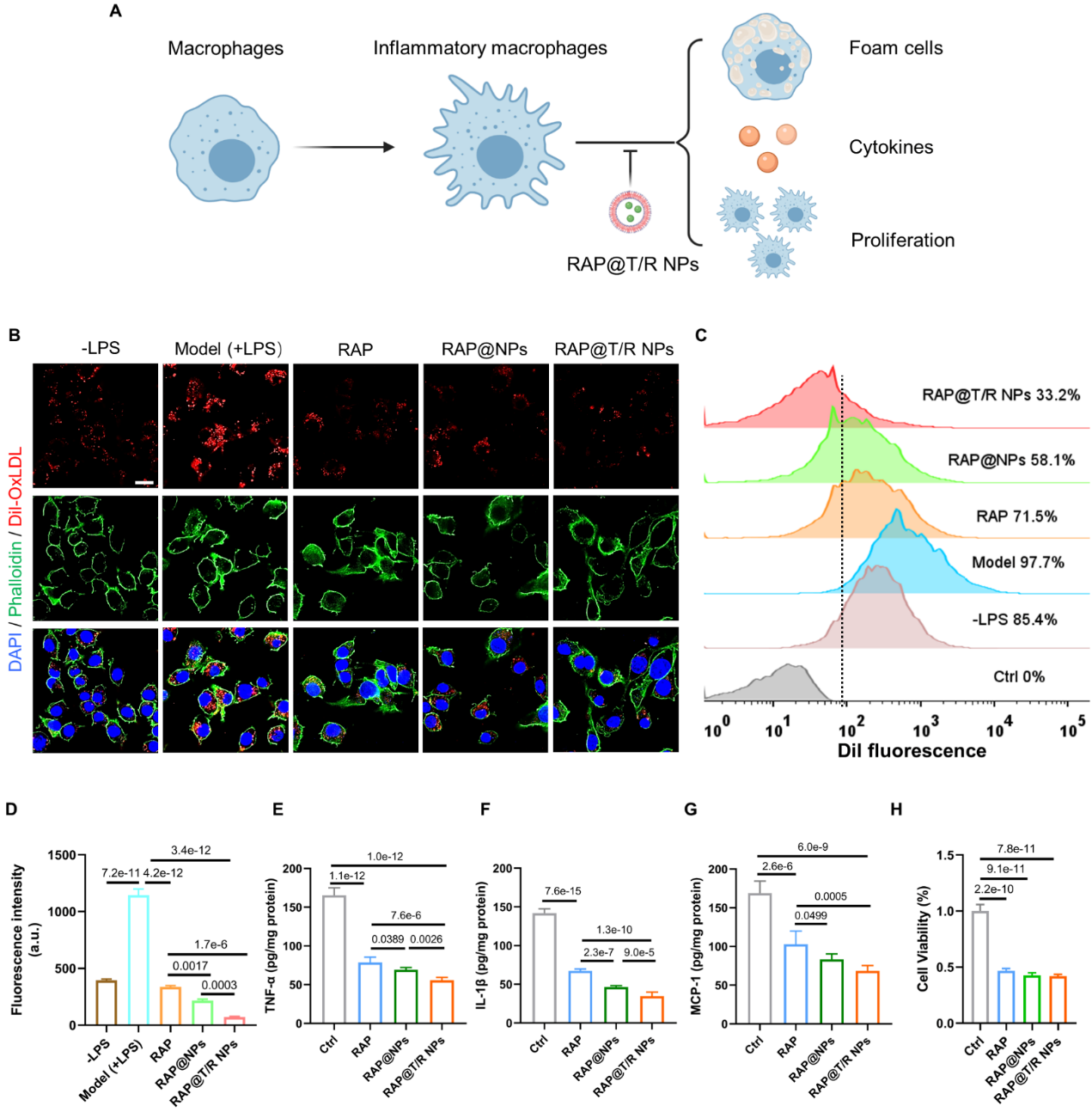
Figure. 5 Evaluation of the therapeutic effect of RAP@T/R NPsin vitro
Thein vivo administrated results of mice showed that RAP@T/R NPs could significantly reduce lipid deposition in the aorta and significantly inhibit the progression of atherosclerosis compared with the control group (Figure. 6). The results of this study are expected to provide new ideas for clinical targeted drug delivery of atherosclerosis.
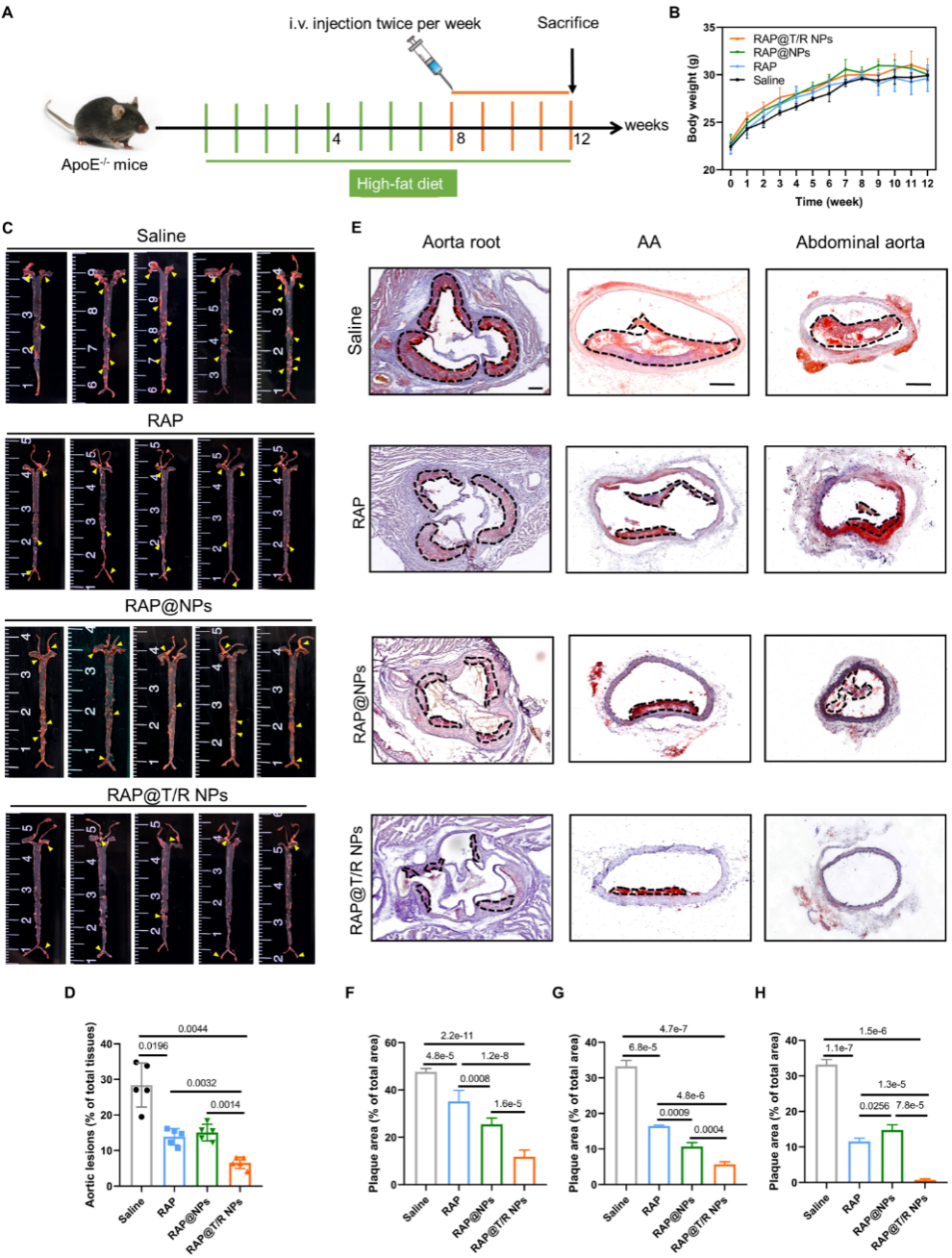
Figure 6. The therapeutic effect of RAP@T/R NPs in vivo
Prof. Xiaoheng Liu and Prof. Shen Yang are the co-corresponding authors of the two papers, Prof. Song Li (UCLA) is the co-correspondingauthor of the article published in the journal Theranostics, and Fang Fei, a 2018 doctoral student in our school, is the first author of the two papers. This research was supported by the National Natural Science Foundation of China (11932014, 32071312).
Original link: https://www.thno.org/v12p4200.htm ;https://doi.org/10.1016/j.gendis.2022.03.020 .